Budesonide Nasal Spray Pregnancy
Budesonide nasal spray pregnancy. For this reason its recommended as the first-choice treatment for allergic rhinitis in pregnant women. I always learned that budesonide rhinocort is safest during pregnancy. Budesonide should be used during pregnancy only if the potential benefits clearly outweigh the risk to the fetus.
Despite the animal findings it would appear that the possibility of fetal harm is remote if the inhaled drug is used during pregnancy. Budesonide has not been shown to be teratogenic in animals when given in high doses by inhalation. Lacking sufficient clinical trials on the use of intranasal corticosteroid sprays in pregnancy we suggest that the intranasal use of fluticasone furoate mometasone and budesonide is safe if they are used at the recommended therapeutic dose after a proper medical evaluation.
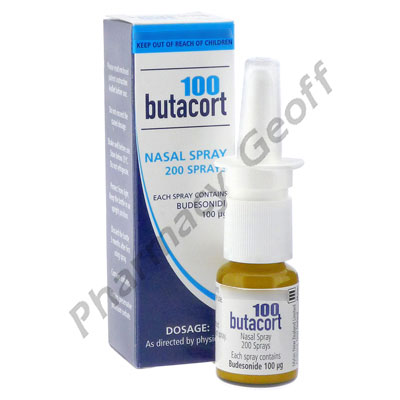
Budesonide nasal spray is available with a prescription as generic budesonide or brand-names Rhinocort Aqua Entocort and Pulmicort. Significant association between the use of intranasal budesonide during pregnancy and overall congenital malformations or overall frequency of cardiovascular defects in the offspring. Show ratings reviews for.
However similar drugs pass into breast milk. However while searching on UpToDate I found a chart describing 1st and 2nd gen nasal steroids. FDA recommends the following in vitro and in vivo studies to establish bioequivalence BE of the test T to the reference R nasal sprays containing budesonide.
Budesonide works by reducing inflammation in the nasal passages. Inhaled budesonide has been assigned to pregnancy category B by the FDA. 1st gen with systemic bioavailability 10-50 and 2nd gen with systemic bioavailabilty.
We have the most information about budesonide use during pregnancy as far as research studies go. It is unknown if nasal budesonide passes into breast milk. Adverse events eg hypoadrenalism observed with systemic corticosteroids in animal reproduction studies.
Budesonide nasal Pregnancy Warnings. Budesonide has not been shown to be teratogenic in animals when given in high doses by inhalation.
We have the most information about budesonide use during pregnancy as far as research studies go.
Inhaled budesonide has been assigned to pregnancy category B by the FDA. Despite the animal findings it would appear that the possibility of fetal harm is remote if the inhaled drug is used during pregnancy. 1st gen with systemic bioavailability 10-50 and 2nd gen with systemic bioavailabilty. Despite the animal findings it would appear that the possibility of fetal harm is remote if the inhaled drug is used during pregnancy. The impact of budesonide on human pregnancy outcomes has been evaluated through assessments of birth registries linked with maternal usage of inhaled budesonide ie PULMICORT TURBUHALER and intranasally administered budesonide ie Budesonide Nasal Spray. We have the most information about budesonide use during pregnancy as far as research studies go. In vitro and in vivo studies. It relieves symptoms such as congestion blocked nose runny nose sneezing and nasal itching. However while searching on UpToDate I found a chart describing 1st and 2nd gen nasal steroids.
In vitro and in vivo studies. FDA recommends the following in vitro and in vivo studies to establish bioequivalence BE of the test T to the reference R nasal sprays containing budesonide. It is unknown if nasal budesonide passes into breast milk. There are no adequate and well-controlled studies in pregnant women. We have the most information about budesonide use during pregnancy as far as research studies go. However while searching on UpToDate I found a chart describing 1st and 2nd gen nasal steroids. Intranasal fluticasone propionate might be a safe option in the absence of other INCS options due to its questionable efficacy during pregnancy.
































Post a Comment for "Budesonide Nasal Spray Pregnancy"